
M3 System
The M3 System Explained

Measure

Monitor
The M3 System® Delivery Module
The Investigative Process and the M3 Protocol
Step 1
Step 2
Step 3
Step 4
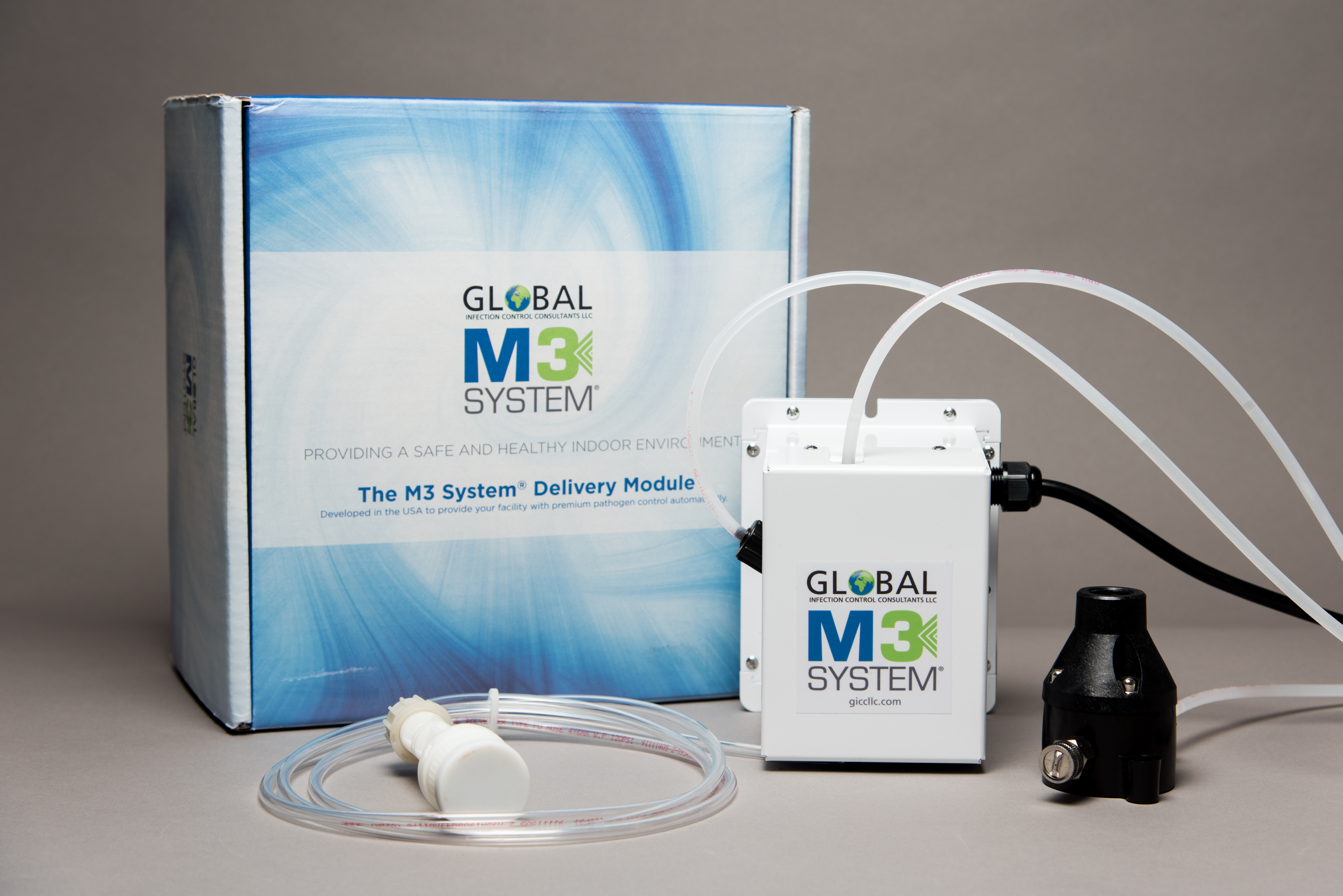
The M3 System® Delivery Module
The M3 System® Delivery Module is the answer to 24/7/365 protection of your facility and occupants, visitors and guests. You can provide a safe and healthy indoor environment when used is combination with Path-Away Anti-Pathogenic Aerosol Solution®. Through the application of our proprietary technology we can adjust and control the frequency and quantity of Path-Away Anti-Pathogenic Aerosol Solution® required to manage pathogen control across a broad spectrum of fungi, bacteria, yeasts and viruses.
After a careful assessment of your needs and based on our assessments of in excess of 5,000+ facilities in more than 16 countries, we can pre-program the module to provide you with the safest, pathogen-controlled environment. The unit is compact, weighing less approximately 10 Lbs. / 4.5 kg, and easily installed on your building HVAC system.
We provide specially designed and manufactured components to complete the system so as to provide maximum efficiency and efficacy with minimum time for project completion.

Path-Away® Fluid Pick-Up Module
Our specially designed Path-Away® Fluid Pick-Up Module operates efficiently by ensuring a steady flow of Path-Away Anti-Pathogenic Aerosol Solution® to The M3 System® Delivery Module.
With no mechanical moving components, the module is virtually trouble free. All of our system components are designed and/or selected for maximum efficiency, ease of installation and assurance of high quality efficacy results.

Path-Away® Fluid Dispersion Nozzle
As a key component of the system package, our Path-Away® Dispersion Nozzle was designed and constructed to maximize the effectiveness of Path-Away Anti-Pathogenic Aerosol Solution® when the system in in operation.
This critical component is internally designed and machined to provide a proper pattern of fluid dispersion at a fixed rate that compensates for pathogen variances that can result from multitude of building construction, conditioned space usage and a broad range of ambient or humanly created changes in the pathogen matrix under scrutiny.
The M3 system was registered with The United States Patent and Trademark Office on September 27, 2011. Registration #4,032,797
US Patent Pending #62/706,137 Technology property of GICC LLC.

